Importing a CDISC ODM XML Document Using a Language Identifier
Overview
This
example imports clinical trials data from a CDISC ODM XML document
by specifying a language identifier with the LANGUAGE= option in the
PROC CDISC statement. By specifying the LANGUAGE= option, PROC CDISC
locates the matching language identifier in the ODM TranslatedText
element.
It creates a SAS format by using the TranslatedText element with a
matching language tag attribute (xml:lang). The created SAS format
is then applied to the data that is imported from the XML document.
The following example
imports the XML document:
<?xml version="1.0" encoding="windows-1252" ?>
<!--
Clinical Data Interchange Standards Consortium (CDISC)
Operational Data Model (ODM) for clinical data interchange
You can learn more about CDISC standards efforts at
http://www.cdisc.org/standards/index.html
-->
<ODM xmlns="http://www.cdisc.org/ns/odm/v1.2"
xmlns:ds="http://www.w3.org/2000/09/xmldsig#"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"
xsi:schemaLocation="http://www.cdisc.org/ns/odm/v1.2 ODM1-2-0.xsd"
ODMVersion="1.2"
FileOID="000-00-0000"
FileType="Snapshot"
Description="testing codelist stuff"
AsOfDateTime="2006-11-03T09:47:53"
CreationDateTime="2006-11-03T09:47:53"
SourceSystem="SAS"
SourceSystemVersion="GENERIC"
>
<Study OID="AStudyOID">
<!--
GlobalVariables is a REQUIRED section in ODM markup
-->
<GlobalVariables>
<StudyName>CODELIST</StudyName>
<StudyDescription>Checking Codelists</StudyDescription>
<ProtocolName>Protocol</ProtocolName>
</GlobalVariables>
<BasicDefinitions />
<!--
Internal ODM markup required metadata
-->
<MetaDataVersion OID="MDV_CODELIST" Name="MDV Codelist">
<Protocol>
<StudyEventRef StudyEventOID="StudyEventOID" OrderNumber="1"
Mandatory="Yes" />
</Protocol>
<StudyEventDef OID="StudyEventOID" Name="Study Event Definition"
Repeating="Yes" Type="Common">
<FormRef FormOID="X" OrderNumber="1" Mandatory="No" />
</StudyEventDef>
<FormDef OID="X" Name="Form Definition" Repeating="Yes">
<ItemGroupRef ItemGroupOID="X" Mandatory="No" />
</FormDef>
<!--
Columns defined in the table
-->
<ItemGroupDef OID="X" Repeating="Yes"
SASDatasetName="X"
Name="ODM Examples"
Comment="Examples of ODM Datatypes">
<ItemRef ItemOID="ID.x" OrderNumber="1" Mandatory="No" />
</ItemGroupDef>
<!--
Column attributes as defined in the table
-->
<ItemDef OID="ID.x" SASFieldName="x" Name="x" DataType="float" Length="12"
SignificantDigits="2" Comment="x">
<CodeListRef CodeListOID="CL.NUMBERS" />
</ItemDef>
<!--
Translation to ODM markup for any PROC FORMAT style
user defined or SAS internal formatting specifications
applied to columns in the table
-->
<CodeList OID="CL.NUMBERS" SASFormatName="NUMBERS" Name="NUMBERS"
DataType="float">
<CodeListItem CodedValue="1">
<Decode>
<TranslatedText xml:lang="de">einz</TranslatedText>
<TranslatedText xml:lang="en">one</TranslatedText>
<TranslatedText xml:lang="es">uno</TranslatedText>
</Decode>
</CodeListItem>
<CodeListItem CodedValue="2">
<Decode>
<TranslatedText xml:lang="de">zwei</TranslatedText>
<TranslatedText xml:lang="en">two</TranslatedText>
<TranslatedText xml:lang="es">dos</TranslatedText>
</Decode>
</CodeListItem>
<CodeListItem CodedValue="3">
<Decode>
<TranslatedText xml:lang="de">drei</TranslatedText>
<TranslatedText xml:lang="en">three</TranslatedText>
<TranslatedText xml:lang="es">tres</TranslatedText>
</Decode>
</CodeListItem>
</CodeList>
</MetaDataVersion>
</Study>
<!--
Administrative metadata
-->
<AdminData />
<!--
Actual data content begins here
This section represents each data record in the table
-->
<ClinicalData StudyOID="AStudyOID" MetaDataVersionOID="MDV_CODELIST">
<SubjectData SubjectKey="001">
<StudyEventData StudyEventOID="StudyEventOID" StudyEventRepeatKey="1">
<FormData FormOID="X" FormRepeatKey="1">
<ItemGroupData ItemGroupOID="X" ItemGroupRepeatKey="1">
<ItemData ItemOID="ID.x" Value="3" />
</ItemGroupData>
</FormData>
</StudyEventData>
</SubjectData>
</ClinicalData>
</ODM>Program
The following SAS program
imports the XML document as a SAS data set:
-
The LIBNAME statement assigns the libref Results to the physical location of the output SAS data set.
-
The FILENAME statement assigns the file reference Xmlinp to the physical location of the input XML document (complete pathname, filename, and file extension) to be imported.
-
The PROC CDISC statement specifies the following:
-
CDISC ODM as the model.
-
File reference Xmlinp, which references the physical location of the input XML document to be imported.
-
FORMATACTIVE=YES to convert CDISC ODM CodeList content in the XML document to SAS formats.
-
FORMATNOREPLACE=NO to replace existing SAS formats in the FORMAT catalog that have the same names as the converted formats.
-
LANGUAGE="DE" to specify a language identifier with a two-letter language code. PROC CDISC locates the DE language identifier in the ODM TranslatedText element. It creates a SAS format by using the TranslatedText element with the matching language tag attribute. The created SAS format is then applied to the data that is imported from the XML document.
-
-
ODMMINIMUMKEYSET=NO in the ODM statement specifies that all KeySet members are written to the output SAS data set. This is the default setting for ODMMINIMUMKEYSET=.
-
The CLINICALDATA statement identifies the output SAS data set (which is Results.Numbers) and specifies the CDISC ODM ItemGroupDef attribute that indicates where the data content in the XML document (which is X) begins.
-
The CONTENTS procedure lists the contents of the output SAS data set.
-
The PRINT procedure prints the rows in the output SAS data set. The VAR statement selects just the X variable.
libname Results 'C:\My Documents\'; 1 filename Xmlinp 'C:\XML\Numbers.xml'; 2 proc cdisc model=odm 3 read=Xmlinp formatactive=yes formatnoreplace=no language="de"; odm odmversion="1.2" odmminimumkeyset=no; 4 clinicaldata out=Results.Numbers sasdatasetname="X"; 5 run; filename Xmlinp clear; proc contents data=Results.Numbers; 6 run; proc print data=Results.Numbers; 7 var x; run; libname Results clear;
Output
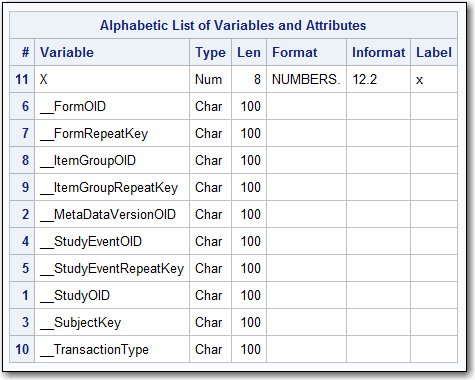
The output from PROC
CONTENTS displays the attributes of each interpreted variable, which
includes the SAS variable X and all KeySet members.
PROC CONTENTS Output for Results.Numbers
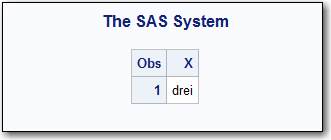
The output from PROC
PRINT lists the value for the imported SAS variable X. The procedure
applies the SAS format NUMBERS, which is created by using the TranslatedText
element with the matching language tag attribute DE. It applies the
SAS format NUMBERS to the data that is imported from the XML document
(which is 3).
The result is the German word
drei
PROC PRINT Output for Variable X
Copyright © SAS Institute Inc. All Rights Reserved.