Importing a CDISC ODM XML Document Using a Language Identifier
Overview
This example
imports clinical trials data from a CDISC ODM XML document by specifying
a language identifier with the LANGUAGE= option in the PROC CDISC
statement. By specifying the LANGUAGE= option, PROC CDISC locates
the matching language identifier in the ODM TranslatedText element.
It creates a SAS format by using the TranslatedText items with a matching
language tag attribute (xml:lang). The created SAS format is then
applied to the data that is imported from the XML document.
<?xml version="1.0" encoding="windows-1252" ?>
<!--
Clinical Data Interchange Standards Consortium (CDISC)
Operational Data Model (ODM) for clinical data interchange
You can learn more about CDISC standards efforts at
http://www.cdisc.org/standards/index.html
-->
<ODM xmlns="http://www.cdisc.org/ns/odm/v1.2"
xmlns:ds="http://www.w3.org/2000/09/xmldsig#"
xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance"
xsi:schemaLocation="http://www.cdisc.org/ns/odm/v1.2 ODM1-2-0.xsd"
ODMVersion="1.2"
FileOID="000-00-0000"
FileType="Snapshot"
Description="testing codelist stuff"
AsOfDateTime="2006-11-03T09:47:53"
CreationDateTime="2006-11-03T09:47:53"
SourceSystem="SAS"
SourceSystemVersion="GENERIC"
>
<Study OID="AStudyOID">
<!--
GlobalVariables is a REQUIRED section in ODM markup
-->
<GlobalVariables>
<StudyName>CODELIST</StudyName>
<StudyDescription>Checking Codelists</StudyDescription>
<ProtocolName>Protocol</ProtocolName>
</GlobalVariables>
<BasicDefinitions />
<!--
Internal ODM markup required metadata
-->
<MetaDataVersion OID="MDV_CODELIST" Name="MDV Codelist">
<Protocol>
<StudyEventRef StudyEventOID="StudyEventOID" OrderNumber="1"
Mandatory="Yes" />
</Protocol>
<StudyEventDef OID="StudyEventOID" Name="Study Event Definition"
Repeating="Yes" Type="Common">
<FormRef FormOID="X" OrderNumber="1" Mandatory="No" />
</StudyEventDef>
<FormDef OID="X" Name="Form Definition" Repeating="Yes">
<ItemGroupRef ItemGroupOID="X" Mandatory="No" />
</FormDef>
<!--
Columns defined in the table
-->
<ItemGroupDef OID="X" Repeating="Yes"
SASDatasetName="X"
Name="ODM Examples"
Comment="Examples of ODM Datatypes">
<ItemRef ItemOID="ID.x" OrderNumber="1" Mandatory="No" />
</ItemGroupDef>
<!--
Column attributes as defined in the table
-->
<ItemDef OID="ID.x" SASFieldName="x" Name="x" DataType="float" Length="12"
SignificantDigits="2" Comment="x">
<CodeListRef CodeListOID="CL.NUMBERS" />
</ItemDef>
<!--
Translation to ODM markup for any PROC FORMAT style
user defined or SAS internal formatting specifications
applied to columns in the table
-->
<CodeList OID="CL.NUMBERS" SASFormatName="NUMBERS" Name="NUMBERS"
DataType="float">
<CodeListItem CodedValue="1">
<Decode>
<TranslatedText xml:lang="de">einz</TranslatedText>
<TranslatedText xml:lang="en">one</TranslatedText>
<TranslatedText xml:lang="es">uno</TranslatedText>
</Decode>
</CodeListItem>
<CodeListItem CodedValue="2">
<Decode>
<TranslatedText xml:lang="de">zwei</TranslatedText>
<TranslatedText xml:lang="en">two</TranslatedText>
<TranslatedText xml:lang="es">dos</TranslatedText>
</Decode>
</CodeListItem>
<CodeListItem CodedValue="3">
<Decode>
<TranslatedText xml:lang="de">drei</TranslatedText>
<TranslatedText xml:lang="en">three</TranslatedText>
<TranslatedText xml:lang="es">tres</TranslatedText>
</Decode>
</CodeListItem>
</CodeList>
</MetaDataVersion>
</Study>
<!--
Administrative metadata
-->
<AdminData />
<!--
Actual data content begins here
This section represents each data record in the table
-->
<ClinicalData StudyOID="AStudyOID" MetaDataVersionOID="MDV_CODELIST">
<SubjectData SubjectKey="001">
<StudyEventData StudyEventOID="StudyEventOID" StudyEventRepeatKey="1">
<FormData FormOID="X" FormRepeatKey="1">
<ItemGroupData ItemGroupOID="X" ItemGroupRepeatKey="1">
<ItemData ItemOID="ID.x" Value="3" />
</ItemGroupData>
</FormData>
</StudyEventData>
</SubjectData>
</ClinicalData>
</ODM>Program
-
-
LANGUAGE="DE" to specify a language identifier with a two-letter language code. PROC CDISC locates the DE language identifier in the ODM TranslatedText element and creates a SAS format by using the TranslatedText items with a matching language tag attribute. The created SAS format is then applied to the data that is imported from the XML document.
libname results 'C:\My Documents\'; 1 filename xmlinp 'C:\XML\numbers.xml'; 2 proc cdisc model=odm 3 read=xmlinp formatactive=yes formatnoreplace=no language="de"; odm odmversion="1.2" odmminimumkeyset=no; 4 clinicaldata out=results.numbers sasdatasetname="X"; 5 run; filename xmlinp clear; proc contents data=results.numbers; 6 run; proc print data=results.numbers; 7 var x; run; libname results clear;
Output
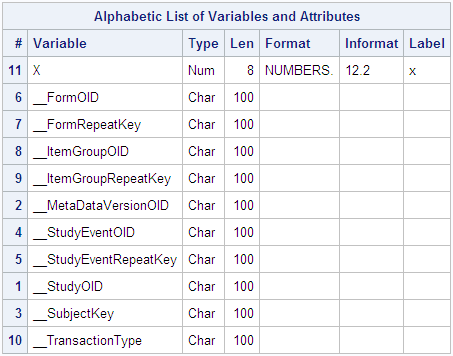
The output from PROC
CONTENTS displays the attributes of each interpreted variable, which
includes the SAS variable X and all KeySet members.
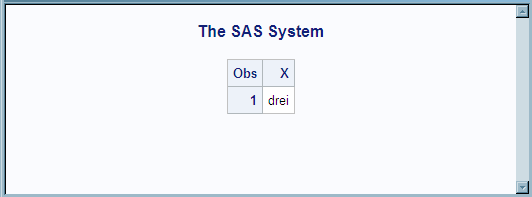
The output from PROC
PRINT lists the value for the imported SAS variable X. The procedure
applies the SAS format NUMBERS, which is created by using the TranslatedText
item with the matching language tag attribute DE. It applies NUMBERS
to the data that is imported from the XML document, which is 3. The
result is the German word drei.